Product Overview
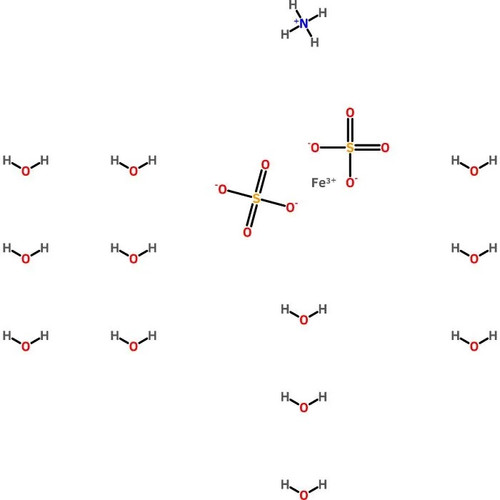
Ferrous Ammonium Sulfate, Hexahydrate, Crystal, Reagent, ACS
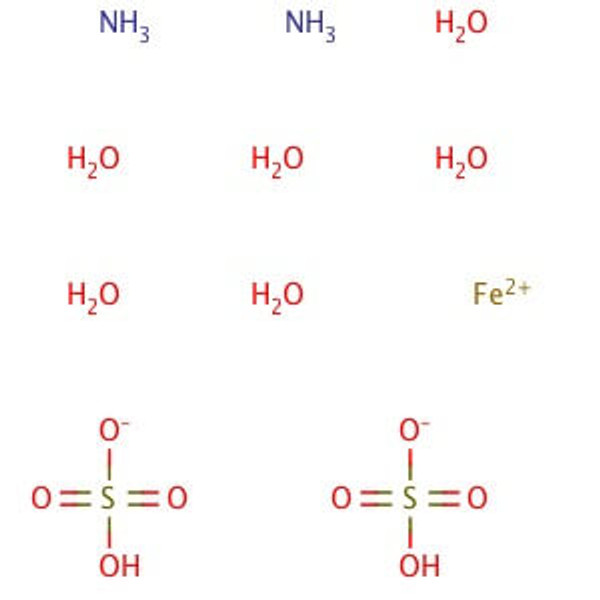
Molecular Formula: (NH4)2Fe(SO4)2•6H2O
CAS Number: 7783-85-9
Molecular Weight: 392.14
Ferrous Ammonium Sulfate, Hexahydrate is an analytical standard which is more resistant to oxidation than iron(II) sulfate. It is often employed as an analytical standard, and has been used in a variety of other applications from nanomaterials to general redox reactions. This product is much less affected by oxygen in the air than iron(II) sulfate, making it more desirable for titration purposes, where iron(II) might be oxidized to iron(III). The oxidation of iron(II) to iron(III) is pH dependent, and the reaction occurs more rapidly at higher pH; Mohr′s Salt lowers the pH of solutions slightly, thereby preventing oxidation from occurring. Reagent ACS grade denotes that this chemical is the highest quality commercially available and that the American Chemical Society has not officially set any specifications for this material. ScienceLab Reagent grade products meet the toughest regulatory standards for quality and purity.
Synonym(s): Ammonium iron(II) sulfate hexahydrate; Mohr′s Salt
| TEST | RESULTS |
|---|---|
| Appearance | Pale blue-green crystals |
| Purity (Titration) | 100.98% |
| Solubility | Soluble in water |
| Content | Ferric ion (Fe3+): < 0.01% |
| Insoluble Matter | 0.004% |
| Calcium | 0.0002% |
| Copper | 0.0004% |
| Magnesium | 0.0001% |
| Manganese | 0.008% |
| Phosphate | < 0.003% |
| Potassium | 0.0004% |
| Sodium | 0.0002% |
| Zinc | 0.0003% |
Shipping Information
Tariff No. 2833300000
Storage Information
LIGHT SENSITIVE, MOISTURE SENSITIVE: Keep tightly closed in light-resistant containers